Qing Dai (QD), commonly known as Indigo naturalis, is a traditional Chinese medicine extracted from leaves and stems of plants like Indigofera tinctoria, Strobilanbthes cusia, O Kuntze, and Polygnonum tinctoium Lour. First mentioned as an anti-inflammatory agent in a foundational 10th-century Chinese medicine textbook, Qing Dai has been used for centuries for heat-cleaning, blood cooling, and detoxification.
Qing Dai is reported to and being researched for the treatment of infectious diseases, skin diseases, tumors, and digestive tract diseases, as well as a number of other diseases.
How QD Relieves Severe Inflammation
Since common pharmaceutical treatments for UC have limited efficacy, there’s been avid interest in complementary treatments such as Qing Dai. Numerous clinical studies show prescriptions containing Qing Dai may be a safe and effective short-term treatment for UC patients failing to respond to pharmaceutical medicine.
Qing Dai has several mechanisms that relieve inflammation and induce mucosal healing in patients with UC. For a start, Qing Dai suppresses the production of proinflammatory cytokines such as TNF-α and IL-6, to name a few. It also inhibits the activation of NF-κB pathways, which triggers inflammatory responses in the body.
Additionally, Qing Dai reduces serum MCP-1 levels and lowers myeloperoxidase (MPO) activity, which is involved in oxidative stress that can lead to chronic inflammation. It also contains ligands for the aryl hydrocarbon receptor and promotes regeneration of the mucosa by inducing the production of interleukin 22.
There’s anecdotal and clinical evidence that Qing Dai is an effective short-term treatment for severe ulcerative colitis, with many patients experiencing rapid remission and mucosal healing.
Please note that Qing Dai specifically should only be taken in strictly recommended doses, for specific conditions, and with thorough follow-ups.
The many anti-inflammatory mechanisms of QD
Qing Dai as a treatment for IBD
Short-term and long-term outcomes of indigo naturalis (QD) treatment for inflammatory bowel disease; a retrospective observational study to investigate the efficacy and safety of QD for induction and maintenance therapy in patients with inflammatory bowel disease.
This survey analyzed data collected from the medical records of patients with IBD who had started Qing Dai (QD) treatment at Kyushu University Hospital. Clinical response and remission rates were measured based on the clinical activity index determined by the Rachmilewitz index, or the Crohn’s disease (CD) activity index. The study included 17 patients with ulcerative colitis and 8 patients with Crohn’s disease.
At 8 weeks, clinical response rates were 94.1%, and remission rates stood at 88.2% in UC patients. For Crohn’s disease patients, clinical response was 37.5%, and remission rates were 25%. Researchers concluded that Qing Dai (QD) shows ‘favorable’ therapeutic efficacy in UC patients and ‘modest’ efficacy in those with Crohn’s Disease.
QD to Treat Ulcerative Colitis
Clinical Efficacy and Safety of Oral Qing-Dai in Patients with Ulcerative Colitis: A Single-Center Open-Label Prospective Study
Researchers at the Keio University Hospital tested the efficacy of oral Qing Dai for inducing remission in patients with moderate ulcerative colitis. 20 patients with moderate to mild UC activity were given oral Indigo naturalis in capsule form twice a day (daily dose: 2g) for 8 weeks.
At week 8, 72% of the subjects reported clinical response, 33% achieved clinical remission, and 61% achieved mucosal healing. Clinical and endoscopic scores, CRP levels, and fecal occult blood results showed significant improvement. The study concluded that Indigo naturalis is effective for inducing remission in patients with moderate UC.
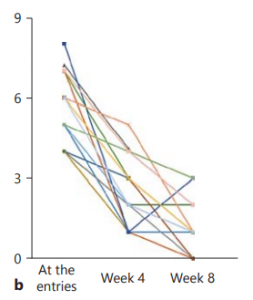
Efficacy of Indigo Naturalis in a Multicenter Randomized Controlled Trial of Patients With Ulcerative Colitis
In 2016, researchers in Japan tested 86 patients with active UC (Mayo score of 6+). The participants were randomized into groups and given a daily dose of 0.5, 1.0, or 2.0 g of Qing Dai (QD) or placebo for 8 weeks. 69.6% of patients in the 0.5 g group showed clinical response, as well as 75% of the 1.0 g group, and 81% of the 2.0 g group.
After 8 weeks, patients in the Qing Dai groups showed significantly higher remission rates than the placebo group. 60% of the 1.0 g group showed mucosal healing, with the other two QD groups close behind. Researchers concluded that 8 weeks of Qing Dai are effective in inducing a clinical response in UC patients.
Efficacy and safety of short-term therapy with indigo naturalis for ulcerative colitis: An investigator-initiated multicenter double-blind clinical trial
This trial aimed to determine the safety and efficacy of Qing Dai (QD) in patients with mild to moderate ulcerative colitis. 46 patients (average age 45) were split into either placebo group or QD group, in which they were administered 5 capsules (500 mg) twice daily for 2 weeks. Researchers took blood tests and measured disease activity on the Lichtiger index before and after treatment. There were no serious adverse events.
The placebo group experienced no change in the Lichtiger index, whereas the QD group showed significant improvement on the Lichtiger index and albumin levels. Researchers concluded that short-term administration of QD is a highly effective and safe short-term treatment.
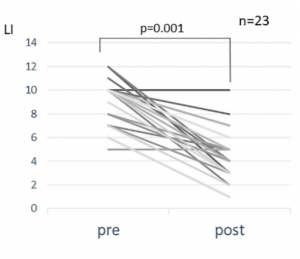
Therapeutic efficacy of the Qing Dai (QD) in patients with intractable ulcerative colitis
This trial observed 9 patients with active ulcerative colitis who had received conventional, pharmaceutical medications and were interested in taking Qing Dai (QD) as an alternative medication. Disease severity was measured with the Clinical Activity Index (CAI), and 5 subjects were endoscopically evaluated according to the Matts grading system. Subjects received 2 g/d of QD orally, along with their prescribed UC medications.
After 4 months of treatment, the CAI score decreased significantly, and patients on prednisolone at the start of treatment were able to discontinue the corticosteroid. The trial concluded that QD has significant clinical and endoscopic efficacy in patients who have failed to respond to conventional medications.
Development of an Indigo Naturalis (QD) Suppository for Topical Induction Therapy in Patients with Ulcerative Colitis`
This open-label, single-center prospective pilot study observed 10 patients with active UC and moderate to severe inflammation from the rectum to the sigmoid colon. Subjects were given a daily QD suppository (50 mg) for 4 weeks.
At the end of the 4-week treatment, 30% achieved clinical remission, and 40% achieved mucosal healing. There was a significant improvement in Mayo rectal bleeding subscores after treatment, and 80% of the patients with baseline Mayo endoscopic subscores in the rectum of 2 achieved mucosal healing. The study concluded that 4 weeks of Qing Dai suppository is a safe treatment for UC patients.
Efficacy of Indigo Naturalis (QD) Therapy for Ulcerative Colitis: A Case Series
This retrospective, observational study evaluated 14 patients with ulcerative colitis who were being treated with QD. After 8 weeks of oral administration, partial Mayo scores decreased from 4 (2-5) to 1.5 (0-4).
50% percent of subjects showed clinical response, and 40% achieved clinical remission. The study concluded that QD is an effective treatment for inducing remission in patients with UC.
Qing-Dai for pediatric ulcerative colitis multicenter survey and systematic review
This trial reviewed the efficacy of Qing-Dai therapy on 107 pediatric UC patients. Within 6 months, 80.2% of the patients had entered clinical remission. The researchers concluded that Qing-Dai was ‘highly effective’ in treating pediatric UC.
Indigo Naturalis (QD) Effective for Treatment of Ulcerative Colitis With Steroid Dependency
In this post hoc subanalysis, researchers analyzed the data from a previous multicentre, double-blind clinical trial that examined the efficacy and safety of QD for inducing clinical response in patients with active ulcerative colitis. Patients were randomized to receive either placebo or QD for 8 weeks. Researchers measured clinical response and the rate of mucosal healing in refractory vs. non-refractory patients.
The rate of clinical response was 77.8% in patients with steroid-dependent disease, 77.5% in patients with previous use of anti–TNF-α, 70.8% in patients with concomitant use of thiopurine, and 76.7% in patients with a Mayo endoscopic score of 9-11. No subjects in the placebo group achieved clinical response.
The QD group saw a significantly higher rate of mucosal healing than the placebo group for patients with steroid-dependent disease. The QD group also showed a decrease in median fecal calprotectin levels at 8 weeks for patients with steroid-dependent disease.
Researchers concluded that 8 weeks of treatment with Qing Dai (QD) is effective in inducing clinical response and mucosal healing, even in patients with steroid-dependent disease or not responding to anti–TNF-α medication.
Treatment with indigo naturalis (Qing Dai) for inflammatory bowel disease and other immune diseases
This single-center, open-label prospective study assessed the efficacy & safety of Indigo naturalis for active ulcerative colitis. 20 patients with mild-to-moderate UC were administered 2g of IN for 8 weeks.
At week 8, 72% of the subjects saw clinical response, with clinical remission at 33%, and mucosal healing at 61%.
